AI TPLC
HealthAIAbout This Course
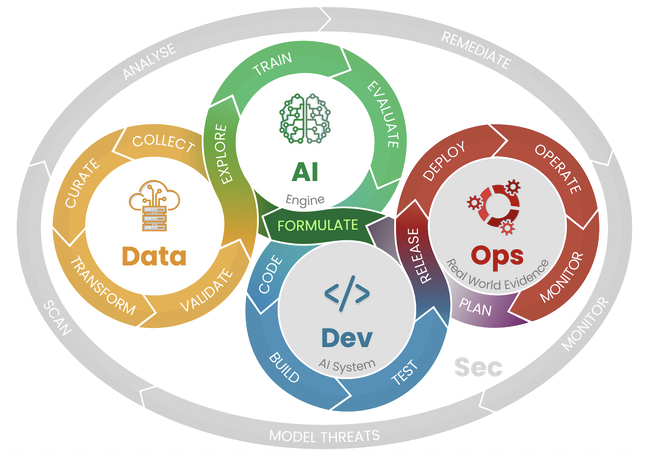
This course introduces the MedTech Total Product Life Cycle (TPLC)—the end-to-end framework that connects discovery, design, development, clinical validation, regulatory compliance, market access, and post-market surveillance. You will learn how decisions made early in product formulation cascade into risk, evidence, and operational requirements later in the lifecycle.
Across interactive lessons and practical checklists, you will map TPLC stages to common standards (e.g., ISO 14971, IEC 62304) and global regulatory pathways (e.g., EU MDR, FDA). We emphasize stakeholder alignment, data and model governance for AI-enabled devices, verification & validation planning, change control (including PCCP/SEP concepts), and real-world performance monitoring. By the end, you will be able to articulate an intended use, identify key risks and controls, and plan compliant, efficient paths from idea to impact.
This course is designed for professionals in R&D, Quality, Regulatory, Clinical, or Product roles, as well as innovators entering the MedTech space. No prior certification is required; a willingness to connect technical, clinical, and regulatory perspectives is the main prerequisite.
Requirements
- Basic familiarity with medical devices, software development, or healthcare operations is helpful but not mandatory.
- Comfort reading standards or guidance documents (we provide summaries and templates).
- Access to a modern web browser and optional spreadsheet tool for templates.
Course Staff
Senior Regulatory Advisor, BioHealth Labs
Marina has over 12 years of experience advising medical device teams on regulatory strategy and evidence generation. She specializes in aligning clinical validation plans with global regulatory expectations for novel medical technologies.
Director, Product & Data Strategy, Orbis Analytics
Jordan brings practical experience in product lifecycle management, data governance, and deploying AI-enabled healthcare solutions. He focuses on bridging engineering, clinical, and regulatory teams to deliver safe and effective products.
Frequently Asked Questions
What web browser should I use?
The Open edX platform works best with current versions of Chrome, Edge, Firefox, or Safari. Please refer to the platform’s list of supported browsers for the most up-to-date information.
Will I receive a certificate?
Learners who complete all graded activities and achieve the passing score will receive a certificate of completion recognizing their understanding of the MedTech TPLC.
How much time should I plan each week?
Expect 2–4 hours per week, including short readings, practical templates, and lightweight quizzes. All materials are self-paced.
Is prior regulatory experience required?
No. We provide concise primers and templates. However, prior exposure to quality systems or software development will help you progress faster.
Accessibility: Images include alt text. Headings and lists are structured for screen readers.